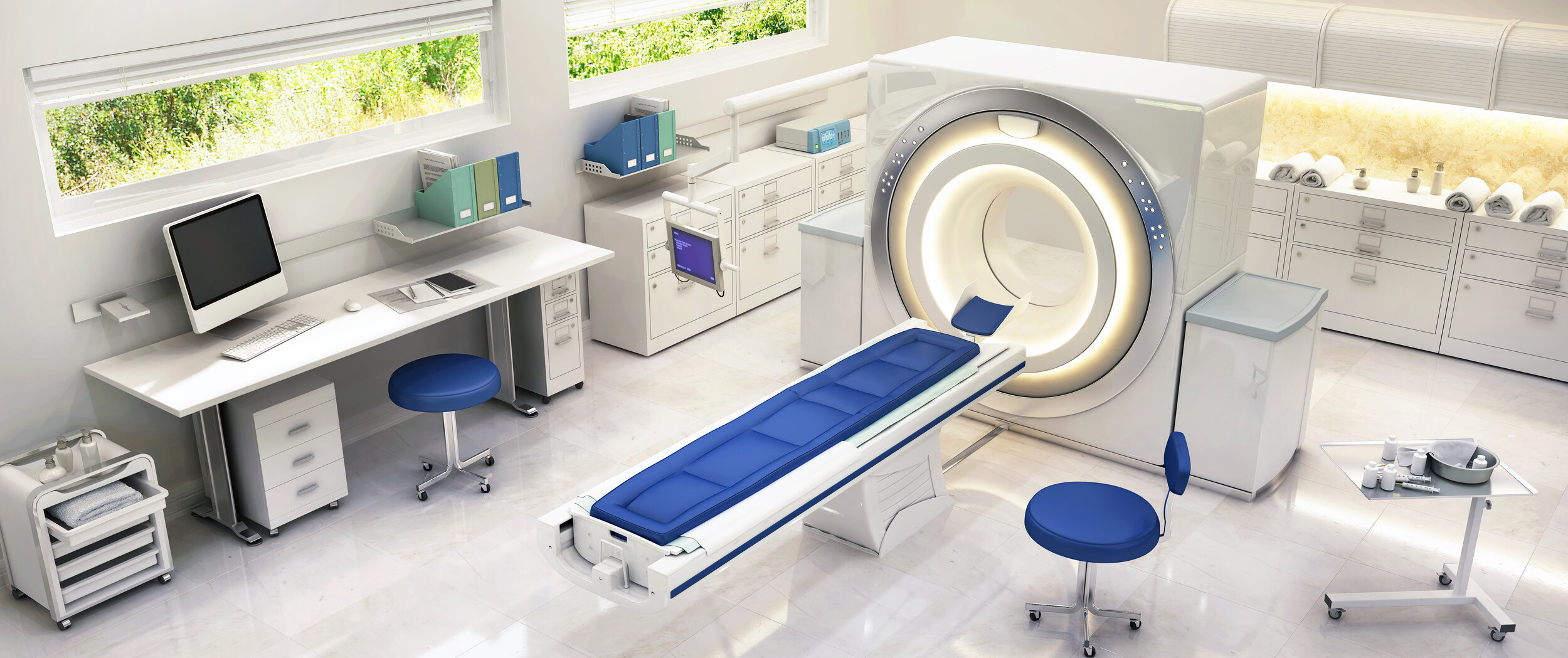
What is MR Conditional?
What is MR Conditional?
The American Society for Testing and Materials (ASTM) International and the Food and Drug Administration (FDA) refer to MR Conditional equipment as items that have no known hazards in a specified MRI environment.
To learn more, please visit the ASTM and FDA websites.
How does Blickman makes their products “MR Conditional”?
Blickman manufactures products primarily from type 304 stainless steel, which generally are regarded as non-magnetic in their original, annealed conditions. Forming the metal tends to bring the magnetizing properties, so we demagnetize the metal via solution annealing and Nitrogen quenching process. Blickman also makes certain to employ, where appropriate non-metal parts and components such as fasteners, hinges, and casters.
What Blickman products are MR Conditional?
Blickman offers the broadest Line of MR Equipment in the Healthcare Market. Our MR Conditional equipment line includes:
Padded stools on rubber tips or on casters.
Foot stools, single-platform or double platform, with or without handrails.
Rolling hampers, with or without plastic lids.
Rolling IV stands, mayo stands, solution stands, and kickbuckets.
Instrument tables and utility tables in a variety of sizes with or without shelves/casters.
Are Blickman’s MR Conditional equipment fully-compliant with the New ASTM Standards?
Yes. All Blickman MR Equipment is suitable for use throughout the entire MR suit, including anywhere within the vicinity of 3.0-Tesla scanners. We also label all our equipment as MR conditional or NOT MR conditional depending on their magnetic features.
How well has Blickman’s MR Conditional Equipment performed?
Since the late-1990s, Blickman products, legendary for high quality, have performed without incident. Blickman’s unblemished record of safety in the imaging industry is owed to faithful adherence to guidelines set by the Center for Devices & Radiological Health (CDHR) - Magnetic Resonance Group, part of the U.S. Food and Drug Administration.
Are Blickman’s MR Conditional Equipment Backed by Blickman’s Limited Lifetime Warranty?
Yes, all Blickman equipment, including MR Conditional equipment, are backed by Blickman’s limited lifetime warranty.
MR Conditional Construction Specs
All products in this Blickman MR Conditional Room brochure meet the revised “MR Conditional” definition as stated in ASTM standard F2503.
The configuration and design safety of the components of these Blickman products in the MR environment were verified in accordance with ASTM standard F2052-06: “Measurement of Magnetically-induced Displacement Force in the MR Environment.”
The “paramagnetic” material property constraints of these Blickman products were determined through testing conducted by an independent lab in accordance with ASTM standard A773/A773M-01, employing a computer automated, soft magnetic, hysteresis graph system.
A low-μ permeability indicator gage, in compliance with ASTM standard A342/A342M, is used for final factory acceptance of these Blickman products.
Identification of these Blickman products conforms to the ASTM standard F2503-05: “Marking Medical Devices and Other Items for Safety in the MR Environment.” MR Conditional Specifications Shipping Information All shipping



